Lorna Wilson and Karen Walsh explain their role as part of a multi-disciplinary team working on a new treatment for certain cancers.
The first high energy NHS-funded proton beam therapy (PBT) service in the UK opened at The Christie NHS Foundation Trust, Manchester, in autumn 2018. As key members of the Allied Health Professional (AHP) multi-disciplinary team (MDT) within PBT, Dietetics and Speech & Language Therapy (SLT) were in a unique position to be part of setting up and setting the standards for a national service and raising the profile of AHPs within cancer care.

The development of the service has provided a valuable learning experience and allowed for the introduction of interdisciplinary working to benefit patient care and experience.
PBT is an advanced form of radiotherapy that uses high-energy protons rather than conventional photons. Like photons, PBT works by damaging the DNA of tumour cells and interfering with their ability to reproduce, but the protons’ Bragg peak leads to a rapid fall-off of dose after the target volume, so tissues after the target receive less dose comparatively (Figure 1).
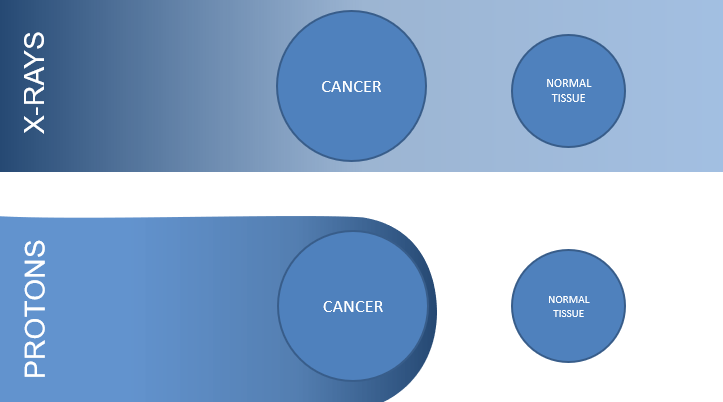
Figure 1: Pictorial depiction of x-ray versus proton dose
The use of PBT can be advantageous when treating tumours located in close proximity to critical structures, for example, the spinal cord, brainstem, brain, eyes or optic nerves. In such cases, treating with conventional radiotherapy may mean that insufficient dose could be delivered to the target volumes without exceeding doses to critical structures and causing unacceptable toxicity. PBT may facilitate dose escalation in these cases.
For other cancers, PBT may reduce the radiation dose to organs at risk (Figure 2), reducing treatment related side effects in the short- and long-term. Further, reducing radiation doses to normal tissues can reduce the risk of secondary malignancies, meaning the greatest potential benefit of PBT may be for children, teenagers and young adults (TYAs).
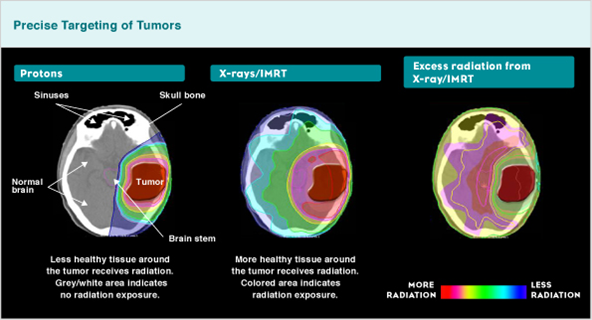
Figure 2: Proton versus x-ray and radiation exposure difference
PBT is routinely commissioned for paediatric and TYA patients and for the treatment of selected cancers of head and neck (H&N), the skull base, sarcomas and central nervous system (CNS). Aside from these indications, it is within the remit of the NHS PBT service to lead on research trials to determine other cancers that may benefit from PBT.
Patients are referred nationally to the proton panel. Once funding has been approved the patient is then invited to a period of assessment (generally across three-to-four days) to allow for scans, planning, alongside clinical assessments. Treatment commences approximately two-to-three weeks after planning with patients who are not from the local area staying in accommodation in Manchester for the duration of their treatment (which can be up to eight weeks).
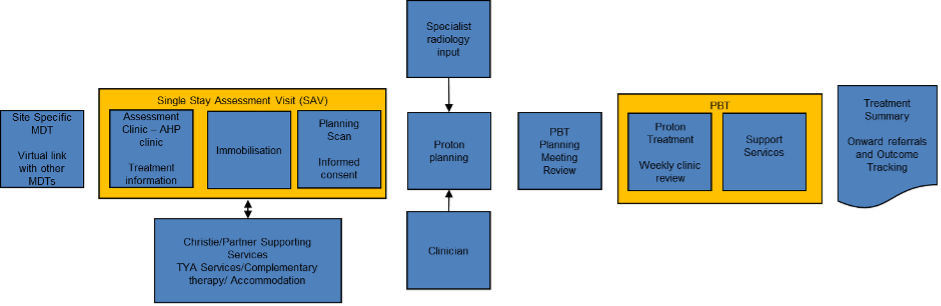
Figure 3: patient pathway
Dietetic and SLT PBT service
Dietetics and SLT sit within an AHP specialist MDT to allow for a coordinated approach to care and treatment.
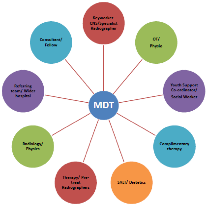
Figure 4: AHP led MDT structure
The challenges experienced during treatment are multifactorial. Disease locality, length and dose of treatment, together with side effects and a prolonged time away from home can have a significant impact on the person’s physical and psychological well-being, quality of life (QOL) and their resilience to tolerate treatment.
To reflect this, the unique MDT (Fig. 4) is available. The team meets weekly and consists of both clinical and non-clinical members to ensure the patients clinical and psycho-social needs are met. Due to the high number of TYA patients, specific TYA members of staff, addiction specialists and complementary therapy are also key members of the MDT.

Figure 5: the RAG rating system
A Red-Amber-Green rating system (figure 5) is used within the MDT meeting to support appropriate discussion and decision making to ensure each patient’s individual needs are being met. This approach has allowed the team to be time efficient and identify the patients with greatest clinical needs.
Focus on H&N and Sarcoma of H&N
All patients under the H&N remit are assessed jointly by Dietetics and SLT. The pre-treatment appointment with the dietitian includes a comprehensive baseline nutritional assessment, looking at current weight and weight history, handgrip strength and a full dietary assessment. There may also be liaison with the patient’s local dietetic department, prior to meeting the patient for the first time for up-to-date handover. The key aim of this assessment is to identify any nutritional problems prior to treatment and advising the patient/carer regarding nutritional support, to help them progress through treatment as smoothly as possible. Each patient is then reviewed weekly or as discussed in the AHP
specialist MDT.
To date, 122 patients aged 16 and above with a H&N diagnosis have been treated with PBT; 93 patients with a H&N cancer have been treated in the Head and Neck service and 29 patients with a H&N sarcoma have been treated in the Sarcoma service.
The PBT H&N team is leading the national TORPEdO trial (TOxicity Reduction using Proton beam therapy for Oropharyngeal cancer). This is a phase III randomised, controlled, multicentre radiation technology trial, which aims to look at the outcomes for patients receiving standard radiotherapy vs. PBT for Oropharyngeal Cancers. Key outcome measures include gastrostomy tube dependence and weight loss at 12 months and tube feeding status at various time points, post PBT completion. This is an exciting project to be part of that will better inform our understanding of the long-term impact of PBT on H&N outcomes and we look forward to reporting on this area in future.
Conclusion
The development of the AHP-led specialist MDT enables a high level of interprofessional working. This ensures a consistent approach, to support each patient to achieve the best possible outcome and improve the patient experience while undergoing PBT.
There have been some novel learning points whilst establishing our service, such as developing our understanding of more unusual H&N diagnoses in line with PBT referral criteria, the sensitivity of weight changes on the PBT planning process and the complexity of supporting TYA patients through treatment. As the UK’s PBT centres develop over the next few years, research trials are a vital component to help decide other suitable candidates for treatment and the best way to use it. These trials will further develop our understanding of treatment side effects ultimately improving patients QOL and rehab potential.




